IDENTIFIATION OF TECHNOLOGIES
RESULTS
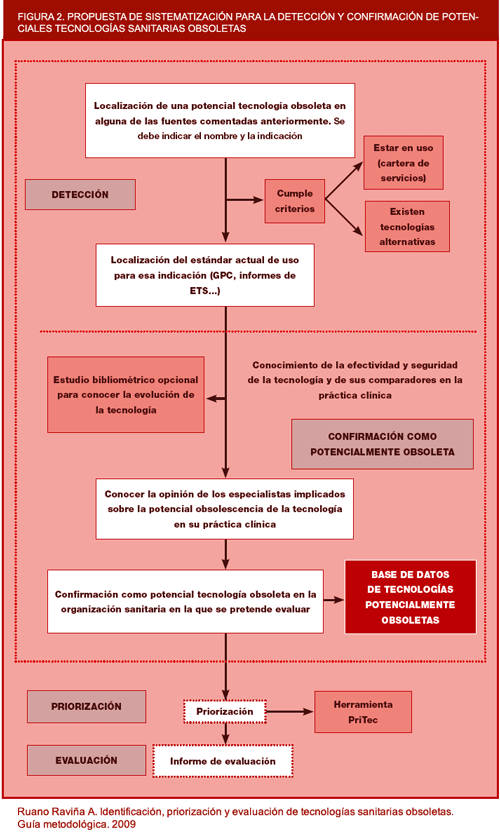
The main sources that can be used for identification of obsolete health technologies are shown below. Figure 2 depicts the steps to be followed for confirmation of technologies as potentially obsolete.
DIRECT SEARCH OF BIOMEDICAL LITERATURE
This would consist of a search of general databases, such as Medline, Embase, Web of Knowledge, etc., and specialised systematic review databases, such as the Cochrane Library Plus, NHS Centre for Reviews and Dissemination (which encompasses the HTA, Database of Abstracts of Reviews of Effectiveness/DARE and the National Health Service Economic Evaluation Database/NHS EED) to locate individual publications on an obsolete technology. In this case, the search could include terms pertaining to obsolescence and the like, or alternatively, terms referring to specific technologies, in order to locate papers on these which might highlight their obsolescence (Appendix I).
REVIEW OF HEALTH TECHNOLOGY ASSESSMENT REPORTS
HTA reports are characterised by systematically reviewing the scientific literature to ascertain the efficacy, effectiveness and safety of new health technologies. Such assessed technologies frequently tend to be diagnostic and therapeutic procedures. The results of these reports evaluate the new procedure against one or more already established procedures, indicating the potential advantages and limitations of the new technologies. HTA reports would therefore be a source capable of detecting potentially obsolete technologies, i.e., those superseded by the new technologies appraised. The added advantage of this type of document is that it is based on robust methodology, so that, in principle, certain aspects concerning the obsolete technology would only have to be completed for a report to be drawn up. To identify such technologies, one would only have to access the web pages of international and national HTA agencies or the INAHTA to locate reports posted by the organisation's member agencies.
EXAMINATION OF NEW AND EMERGING TECHNOLOGIES DATABASE
The clearest example of these databases is EuroScan (http://www.euroscan.bham.ac.uk/). EuroScan is an international organisation whose designated aim is to compile all the dossiers on emerging health technologies published by its member agencies. To this end, it keeps a database with partially restricted access to members (though with 65% accessible by the public). When information on an emerging technology is entered into this database, a number of fields are covered, and one of these refers to the new or emerging technology's function in its respective health-care system. Hence, indication must be given as to whether the new technology is an alternative to, complements or replaces an existing technology. In addition, the current treatment standard for the condition to which the new technology is applicable is specified. Accordingly, EuroScan, like other similar sources, such as the GENTecS group (Grupo de Evaluación de Nuevas Tecnologías Sanitarias del Sistema Nacional de Salud), could be a source of detection for obsolete health technologies, though the information on the above-mentioned fields is not always covered (it is not mandatory), and sometimes no indication is given as to whether or not the new technology replaces an existing technology. This is fundamentally due to the stage of development reached by the new technology in question, since in the early stages it is difficult to assign a specific function for the new or emerging technologies (29).
For instance, of the 1,129 entries recorded by EuroScan until 15 May 2008, a total of 274 (24.3%) had been designed as replacements. Of the technologies designated as replacements, almost half corresponded to drugs and a quarter to devices. Of the remainder, 14% were procedures and 9% were diagnostic tests (29). Other databases which contain information on new and emerging technologies and may therefore serve for identifying obsolete health technologies are Hayes (USA), the Emergency Care Research Institute (ECRI) (USA) and the Australian Safety and Efficacy Register of Interventional Procedures-Surgical (ASERNIP-S).
One possible strategy for identifying obsolete health technologies through emerging technology databases would be as follows: 1) start with the oldest technologies (introduced in 2000); 2) seek the most recent standard treatment (clinical practice guidelines, HTA reports, systematic reviews); 3) conduct a brief bibliometric study to ascertain the trend in the literature published on the potentially obsolete technology; 4) corroborate the situation of these technologies in the service portfolio; and, 5) ascertain clinicians' opinions about the technology. Once this has been done, the technology would be deemed potentially obsolete and a systematic review would be undertaken to confirm this. This procedure could be used in all possible methods of detection of potentially obsolete technologies and compared to existing treatment recommendations.
DIRECT COMUNICATION WITH CLINICIANS AND RESEARCHERS. OBSOLTETE HEALTH TECHNOLOGY DETECTION NETWORKS.
In the same way as many agencies have emerging health technology detection networks made up of health professionals who report the appearance of any potential new technology to the agencies (DETECTA-t, SorTek, Síntesis), these networks could be used to identify obsolete health technologies. Another possibility would be for potentially obsolete technologies to be identified by using the GENTecS alert networks, which encompass and co-ordinate Spanish agencies with emerging technology detection systems. Whenever one of the members of a detection network (or even any professional in a health organisation) believed that there was a potentially obsolete technology for a specific indication, he/she could report it to the relevant agency (or to GENTecS), which would assess this report (or notify the agencies, in the case of GENTecS). These networks could also be used in cases where agencies detect potentially obsolete technologies and consult the network's members (belonging to the speciality area in question) to confirm the potential obsolescence of such technologies. In this case, the detection network would be acting proactively.
Networks of this type have shown their usefulness when asked to identify obsolete health technologies. At a request from Osteba concerning the identification of obsolete technologies, 65 of the participants consulted identified 12 potentially obsolete technologies in two rounds, and two of these 12 potentially obsolete technologies had been analysed in a systematic review. These consultations are being regularly conducted, and a standardised procedure for identification of potentially obsolete technologies has been proposed in the Basque Country Autonomous Region.
DATABASES ARISING UNDER PREVALING LEGISLATION
At present, Spanish health regulations envisage the exclusion of health technologies (RD 1030/2006) (30). Furthermore, the Ministry of Health & Consumer Affairs Order (Orden SCO) 3422/2007, which establishes the mechanisms for updating the NHS common service portfolio (4) lays down that, when the inclusion of a new technology is sought, the alternatives, if any, must be specified, along with an indication as to whether the new technology replaces them totally, partially or, in contrast, not at all. Moreover, some regional, e.g. Galician (5), regulations lay down that any new technology proposed must indicate, "any modifications that will ensue in the clinical management of patients with respect to currently available alternatives".
Accordingly, contact with all the secretariats of committees tasked with the introduction of new technologies, at both a national and regional level, could serve to identify potentially obsolete technologies. Furthermore, contact could also be made with hospital committees that usually use the GANT guideline (7) for the introduction of new technologies, or that use the GuNFT guideline (31) or other guidelines drawn up by hospital centres to ascertain which technologies are being proposed for replacement or withdrawal of funding respectively.
In order for this potentially obsolete technology identification procedure to function properly, it would be important to have a database with information on HTA hospital committees/units or secretariats of performance committees which use the above-mentioned health technology introduction or exclusion systems.
A similar approach would be to identify technologies that are withdrawn from clinical practice by a range of bodies, such as the US Food and Drug Administration (FDA), or European or Spanish Drug Agencies which generally issue safety alerts for withdrawal of medications.
Figure 2. Proposed systematisation of the detection and confirmation of potentially obsolete health technologies.
From the above figure, it can be deduced that there could be three stages in the identification and confirmation of obsolete health technologies. An initial stage would consist of preliminary identification of probably obsolete health technologies by means of any of the above-described sources of detection. Then, confirmation would be needed that the "probably obsolete" health technology did fulfil the criteria for being defined as such and was therefore "potentially obsolete". Classification of a health technology as potentially obsolete could be done by means of a brief document in the format of a technical dossier. Confirmation of such potentially obsolete technologies as truly obsolete would have to be accompanied by a far longer and more rigorous report, based on a systematic review (described in Section 4 below).