GENERAL COMMENTS AND GUIDELINE LIMITATIONS
This guide seeks to cover a methodological gap in health technology assessment. Until now there has been no rigorous approach to assessment of potentially obsolete health technologies, despite the fact that its relevance has been acknowledged in health organisations.
A very important aspect which should not be overlooked when evaluating a potentially obsolete health technology is that the medical bibliography usually only considers the potential benefits of a new versus a standard technology (whether or not obsolete). This is logical, since it is always the view that new technologies ought to surpass and supersede the best alternative available or, at least, the standard technique (principle of beneficence), and the magnitude of this improvement is examined. Yet, it is also true that this implies that the technology already in place would show a loss of benefit compared with the new technology. This is the aspect which must be considered when evaluating obsolete technologies. An added problem is that technologies used for comparison often vary over time, something that might occasionally render assessment of the results of an obsolete technology versus the current treatment standard very difficult, since there may be few studies that compare these directly.
Indeed, as pointed out by Pearson and Littlejohns (12), the principal problem for assessing an obsolete technology is the dearth of data, and these authors also note that there is no technology that does not at least enjoy limited -but all the same, enthusiastic- support. Accordingly, this calls for a very rigorous approach to the evidence (based on robust data from sound studies) for taking any decision which entails reducing or eliminating the funding of certain technologies.
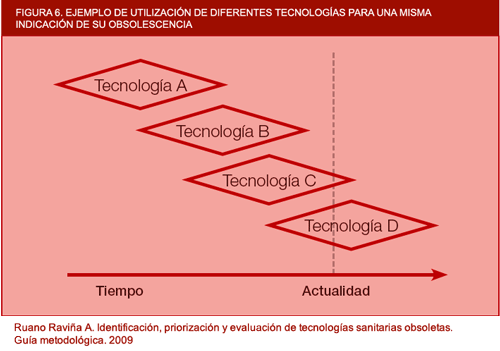
Figure 6. Example of use of different technologies for a single indication. de su obsolescencia
Another important point is that this guide does not formally address the financial aspects linked to obsolete technologies. Despite the fact of there being a costs and organisation section in the proposed report model, it is not the aim of this guide to conduct a comprehensive analysis of the possible financial or organisational repercussions of an obsolete technology's withdrawal. Although we acknowledge that this topic is important, the essential element that should determine the withdrawal of funding from any obsolete technology is its lack of effectiveness or safety. At all events, it would be extremely interesting if reports on obsolete technologies were to incorporate a somewhat more detailed section on their financial repercussions, though another solution would be for this type of analysis to be performed for specific obsolete technologies or obsolete technologies which are as effective and safe as current ones but more expensive in financial or organisational terms.
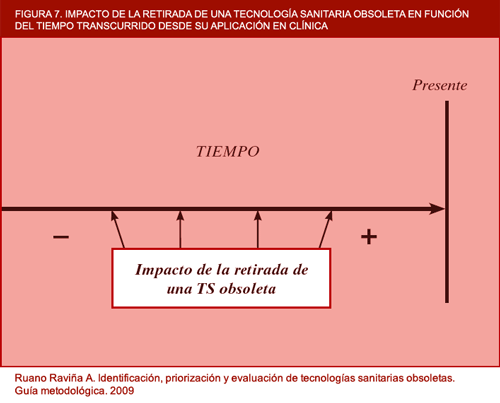
The financial aspect of obsolete technology assessment is an open field and one that ought to be developed in the near future. Institutions such as the NICE have cost-effectiveness thresholds for new versus already established technologies (11) and perhaps these thresholds could also be used for the characterisation of certain technologies as obsolete, though there may be other factors that influence health technology obsolescence to a greater degree. For instance, Buxton has indicated the need to know the cost-effectiveness of technologies which should be phased out by the NICE in the near future, and that this institution should locate cost-ineffective technologies in order to recommend disinvestment from them. He also makes the point that this need is particularly important when the National Health Service is emerging from a period of 7 years of budgetary strength and is going through a lean period (NOTE: when the author wrote the above, the current world financial crisis had not yet begun). He notes that the NHS must assimilate the idea of active disinvestment from cost-ineffective activities. In conclusion, it would perhaps be interesting to draw up a series of criteria defining when financial assessment of potentially obsolete health technologies is called for, since this will not always be necessary; and it would likewise be interesting to draw up a brief methodological guide that would indicate how this type of assessment was to be made. Elshaug et al have also recently proposed prioritisation criteria for more detailed assessment targeted at disinvestment from health technologies, while the GuNFT guideline, drawn up by Osteba, proposes criteria for undertaking this process in an organised, systIt is evident that the detection of an obsolete health technology is closely related to the time that has elapsed since it was introduced into clinical practice, as can be seen in figure 7. The more time that has elapsed since its introduction, the greater the likelihood of the technology being obsolete. Nevertheless, this type of technology is bound to have been displaced by another more efficient technology, and it is more than likely that it is no longer used. Detection and assessment of such technologies would be a process that would consume agency resources and, in return, afford little benefit.
Presumably, the true objective of an obsolete health technology characterization are those technologies which are currently being used in clinical practice and which may even have been introduced into health-care system just a few years previously. Detection and exclusion of such technologies would be expected to have a great impact on the health-care system and make way for a major reallocation of resources (a reinvestment taken in positive terms). Notwithstanding this, detection of these types of technologies is complex owing to a number of factors. In the first place, if they have been applied for only a short time, it is possible that few studies may have been published and, though the evidence might point to their having a diminished effect, this would not be enough to recommend their exclusion. Furthermore, any studies which have been published since the new technology was introduced and which assess other technologies are bound to have used earlier comparators to enhance the effect of the new technology, thus again rendering comparison complex. At all events, agencies would have to identify potentially obsolete technologies for prioritisation and assessment purposes, making every effort to ensure that these were relevant in terms of the potential impact (including, logically, impact on patients) to be generated by their withdrawal from health organisations.
Figure 7. Impact of withdrawal of obsolete health technologies according to time elapsed since their application in clinical practice (in-house).
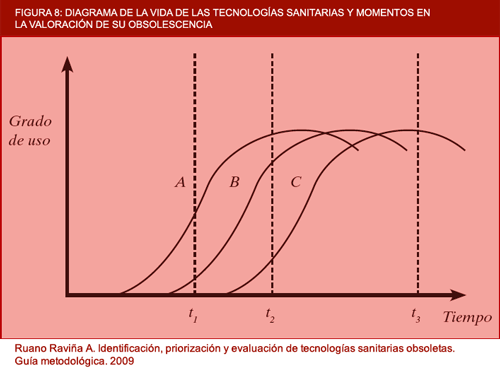
Figure 8. Diagram of the life of health technologies and points in time in the assessment of their obsolescence (in-house).
In the above figure, a number of situations show the obsolescence of different technologies to be assessed. A, B and C are different technologies for treating the same condition, which have arisen at different moments in time. Assuming that the newest technologies are better than their predecessors in terms of efficacy and safety, it can be deduced that, at point in time, t2, A and B would be obsolete with respect to C, and an important benefit would be obtained if they were withdrawn from clinical practice. Technology A has already reached a plateau and B is about to level off and reach its plateau. At point in time, t1, assessment of the technologies would show that A was obsolete with respect to B, though it would perhaps be premature for A to be withdrawn because there are still aspects of B to be clarified, since this latter technology was introduced into clinical practice a very short time previously. The most unfavourable point in time for assessing technologies A and B as potentially obsolete would be t3, since it is clear that both have fallen into disuse and are no longer relied upon, so that the benefit of classifying them as definitely obsolete would be negligible.
Just as there is a systematic procedure in Spain and in autonomous regions such as Galicia for introducing health technologies and updating the service portfolio, it would be of interest if the foreseeable withdrawal of health technologies that became obsolete as they were displaced by technologies being introduced into clinical practice, could be planned in the same way. For instance, the introduction of Positron Emission Tomography-Computerised Tomography (PET-CT) for evaluation of solitary pulmonary nodules will progressively displace the use of CT on an individual basis, and the use of this latter technology in the detection of pulmonary nodules will then grow obsolete as PET-CT becomes increasingly available in hospitals.
Another aspect that warrants discussion is the unpopularity of defining certain technologies as obsolete. There may be manufacturers who are not in agreement with the classification of their products as obsolete, and health decision-making may also become reticent, since there is the danger that disinvestment may be made from services in which there is no strong leadership rather than from those which are genuinely less safe or effective (11). As a NICE document indicates, "there will be many clinicians and perhaps groups of persons that prefer the status quo, regardless what the evidence may say", thereby underscoring the fact that a crucial aspect for preparing the ground will be communication with the different groups to prepare them for disinvestment. A further problem could be that, despite there being more effective technologies, these cannot be implemented for resource-related or organisational problems, as has happened in some health systems on cobalt pumps being gradually replaced by linear accelerators for radiotherapy treatments.
Within the context of obsolete technology data sources, searching scientific literature proves complex and depends on the search terms used. Time is required in selecting the information, and the type of information varies depending on its origin and the type of publication. For the introduction of health technologies, the clinical trial is the benchmark research study. Nonetheless, this type of study sometimes responds to a complex methodological design and calls for major financial investment. Clinical trials which involve a potentially obsolete technology are all but non-existent, unless the technology to which this is being compared has recently come onto the market. Withdrawal of medications or suspension of marketing and sale can be simpler than the withdrawal of a given health technology, since in general the studies available are more robust (they are almost always clinical trials) and the medications are indicated for very specific conditions. This does not mean to say, however, that similar regulatory mechanisms may not have to be implemented for obsolete or ineffective health technologies.
Moreover, detection and classification of a health technology as obsolete would be incomplete if this information were not transferred to clinical practice to ensure that patient health was protected and health care ultimately improved. Such obsolete technologies ought to be excluded from clinical practice by a statutory procedure which provides for this, and so be in a position to benefit from the legal coverage offered by the Spanish legislation in force (30).