INTRODUCTION
Health technology assessment (HTA) has traditionally been used to ascertain the efficacy, effectiveness and safety of new health technologies and to establish standards and recommendations in clinical practice. Although new procedures do not inevitably improve population's health, it is true that, with time, technologies which have already been implemented are progressively superseded by newly emerging ones. Consequently, some health technologies (HTs) will always be in the process of becoming obsolete, either because there are other technologies that are more effective, safer and cheaper or because other technologies combine some of these characteristics to a lesser or greater degree. There may be other aspects that cause technologies to fall into disuse, such as ethical aspects or the preferences of professionals, patients or society in general, though these are not such explicit reasons as those cited above for health organisations, which fundamentally focus on the effectiveness, safety and cost of health technologies. Nevertheless, when it comes to delivery of health services, institutions are increasingly taking patient and professional preferences into account, so that it is foreseeable that patient preferences may soon come to play a decisive role, and this will then contribute to the obsolescence of technologies.
Health technology is defined as the set of medications, devices and medical or surgical procedures used in health care, along with organisational and support systems within which such care is provided, whereas health technology assessment is the form of research that examines the clinical, financial and social consequences, including any short- and medium-term consequences, as well as any direct, indirect, desired and undesired effects stemming from the use of a technology (1). It is easy to fit assessment of potentially obsolete health technologies into both of these definitions, since in the case of obsolete health technology, the tag, "and which has been superseded by other technologies", would only have to be added to the preceding definition, and the same is applicable to the definition of assessment of potentially obsolete health technologies.
Many HTA agencies have emerging health technology detection systems, aimed at ascertaining the potential impact that a new health technology might have on the health system. The purpose of these systems is to ensure that health-care organisations can anticipate and be prepared for decision-making on the new HT's possible implementation. Almost all programmes designed to detect these technologies rely on different data sources for their identification, such as scientific literature, health professionals, congresses, etc. The products deriving from early identification of health technologies are known as "brief reports", "early warnings", "alerts", etc., and are brief documents that succinctly analyse these new technologies, focusing on their foreseeable impact on health care and organisation of health resources.
Just as there is a system for early detection and assessment of emerging technologies, there should be another that would enable detection and assessment of health technologies which may have become obsolete and ought to be evaluated using a mechanism similar to emerging health technology detection programmes. In addition to a mechanism for identifying technologies, an obsolete technology detection system would also need a prioritisation system, since there will be many obsolete technologies and a common methodology for assessment of such technologies. At all events, all potentially obsolete technologies should be assessed in accordance with standard HTA methodology, so that, provided the necessary requirements are met, these can be subsequently classed as obsolete technologies. As is the case with any assessment of a health technology, assessment of a potentially obsolete health technology must be irreproachable in its scientific rigour.
The first and foremost advantage of a system of these characteristics would be to furnish evidence for the possible withdrawal from clinical practice of procedures, devices, organisational systems, surgical approaches, etc., which gave rise to more adverse (or more severe) effects than did current treatment standards (e.g., cobalt pump versus linear accelerators in radiotherapy treatments). This would ensure that patients received far safer treatments. Adverse effects must be understood to mean, not only those that directly affect patients, but also those that affect the environment or healthy individuals (e.g., in screening programmes). The second advantage would be the withdrawal of technologies that were less effective than current treatment standards. This would translate as a direct benefit for the National Health System as well as a clinical benefit for patients. It would also reduce the time devoted to such obsolete health resources and make it available for more suitable health care. Thirdly, it would afford the advantage of optimising resources and allocation of health investment. Time devoted to these technologies would be more efficiently employed. However, there is nothing to guarantee that all this would necessarily mean a saving in health expenditure, since many of the new technologies are likely to be more expensive than the technologies classified as obsolete.
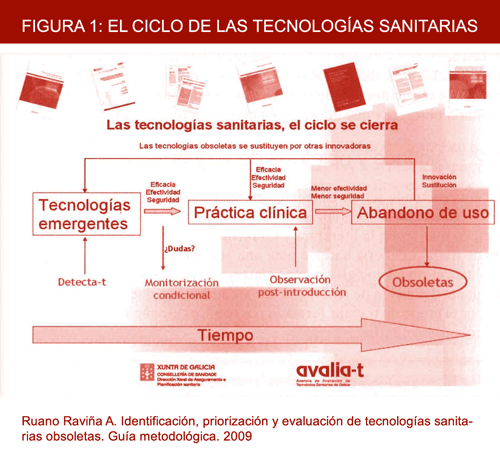
Implementation of a system designed to identify, prioritise and assess obsolete health technologies will enable the life cycle of any given health technology to be brought to an end. This cycle can be seen in Figure 1 and highlights the need to cover the gap that has existed until now in detecting and recommending the exclusion from clinical practice of technologies that have been amply superseded by others.
Figure 1. Health technology cycle.